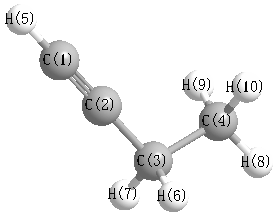
.
| squib |
reference |
DOI |
| 1970COX/PIL |
J.D. Cox, G. Pilcher, "Thermochemistry of Organic and Organometallic Compounds" Academic Press, London, 1970 |
10.1002/bbpc.19700740727 |
| 1983Lan/Sue:210 |
BM Landsberg, RD Suenram "1-BUTYNE MICROWAVE-SPECTRUM, BARRIER TO INTERNAL-ROTATION, AND MOLECULAR DIPOLE-MOMENT" J. Mol. Spect. 98(1) 210-220, 1983 |
10.1016/0022-2852(83)90215-1 |
| 1989Gui/Dur:101 |
GA Guirgis, JR Durig, S Bell "Torsional Transitions and Barrier to Internal rotation of 1-butyne" J. Mol. Struct. 196 (1989) 101-111 |
10.1016/0022-2860(89)85008-2 |
| 1995Bas/Bak:77 |
Bastiansen, Bakken, Kloster-Jensen, Samdal, and Trætteberg. A theoretical and experimental study of the molecular structure of 1-butyne. J. Mol. Struct. Vol. 352/353. pgs. 77-85. |
10.1016/0022-2860(94)08527-O |
| HCP_Polar |
TM Miller, Handbook of Chemistry and Physics Online (http://hbcponline.com/faces/documents/10_04/10_04_0001.xhtml) |
|
| NISThydrocarbon |
NIST Hydrocarbon spectral database (http://www.physics.nist.gov/PhysRefData/MolSpec/Hydro/index.html) |
10.18434/T4PC70 |
| TRC |
Frenkel, M; Marsh, K.N.; Wilhoit, R.C.; Kabo, G.J.; Roganov, G.N.,Thermodynamics of Organic Compounds in the Gas State,Thermodynamics Research Center, College Station, TX, 1994 |
|
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |