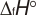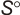Geometric Data

Point Group D∞h
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description |
Value |
unc. |
Connectivity |
Reference |
Comment |
| Atom 1 |
Atom 2 |
Atom 3 |
Atom 4 |
| rCuCu |
2.220 |
|
1 |
2 |
|
|
webbook |
re |
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| Cu1 |
0.0000 |
0.0000 |
0.0000 |
| Cu2 |
0.0000 |
0.0000 |
2.2197 |
Atom - Atom Distances 
Distances in Å
| |
Cu1 |
Cu2 |
| Cu1 |
|
2.2197 |
| Cu2 |
2.2197 |
|
Calculated geometries
for Cu
2 (Copper diatomic).
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
Connectivity