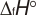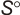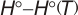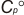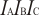Geometric Data
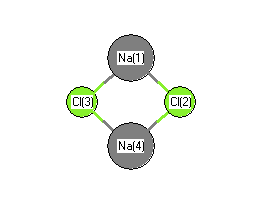
Point Group D2h
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| Na1 |
0.0000 |
0.0000 |
0.0000 |
| Cl2 |
1.9990 |
-1.6366 |
0.0000 |
| Cl3 |
-1.9990 |
-1.6366 |
0.0000 |
| Na4 |
0.0000 |
-3.2732 |
0.0000 |
Atom - Atom Distances 
Distances in Å
| |
Na1 |
Cl2 |
Cl3 |
Na4 |
| Na1 |
|
2.5835 | 2.5835 | 3.2732 |
| Cl2 |
2.5835 |
|
3.9980 | 2.5835 |
| Cl3 |
2.5835 | 3.9980 |
|
2.5835 |
| Na4 |
3.2732 | 2.5835 | 2.5835 |
|
Calculated geometries
for Na
2Cl
2 (Disodium dichloride).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| Na1 |
Cl2 |
Na4 |
78.615 |
|
Na1 |
Cl3 |
Na4 |
78.615 |
| Cl2 |
Na1 |
Cl3 |
101.385 |
|
Cl2 |
Na4 |
Cl3 |
101.385 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
Connectivity
| Atom 1 |
Atom 2 |
| Na1 |
Cl2 |
| Na1 |
Cl3 |
| Cl2 |
Na4 |
| Cl3 |
Na4 |