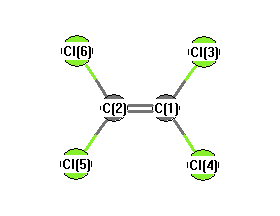
.
| squib |
reference |
DOI |
| 1976Hellwege(II/7) |
Hellwege, KH and AM Hellwege (ed.). Landolt-Bornstein: Group II: Atomic and Molecular Physics Volume 7: Structure Data of Free Polyatomic Molecules. Springer-Verlag. Berlin. 1976. |
|
| 2002Man:123 |
JA Manion "Evaluated Enthalpies of Formation of the Stable Closed Shell C1 and C2 Chlorinated Hydrocarbons" J. Phys. Chem. Ref. Data 31(1), 123-172, 2002 |
10.1063/1.1420703 |
| Gurvich |
Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub. Co., New York, 1989 |
|
| NSRDS-NBS10 |
R. D. Nelson Jr., D. R. Lide, A. A. Maryott "Selected Values of electric dipole moments for molecules in the gas phase" NSRDS-NBS10, 1967 |
10.6028/NBS.NSRDS.10 |
| Shim |
Shimanouchi, T. , Tables of Molecular Vibrational Frequencies, Consolidated Volu |
10.6028/NBS.NSRDS.39 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |