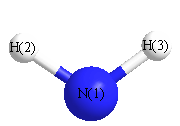
.
| squib |
reference |
DOI |
| 1966Herzberg |
Herzberg, G., Electronic spectra and electronic structure of polyatomic molecules,Van Nostrand,New York, 1966 |
|
| 1979HUB/HER |
Huber, K.P.; Herzberg, G., Molecular Spectra and Molecular Structure. IV. Constants of Diatomic Molecules, Van Nostrand Reinhold Co., 1979 |
10.1007/978-1-4757-0961-2 |
| 2005Rus/Bog:573 |
B Ruscic, JE Boggs, A Burcat, AG Csaszar, J Demaison, R Janoschek, JML Martin, ML Morton, MJ Rossi, JF Stanton, PG Szalay, PR Westmoreland, F Zabel, T Berces "IUPAC Critical Evaluation of Thermochemical Properties of Selected Radicals. Part I" J. Phys. Chem. Ref. Data, Vol. 34, No. 2, 2005, 573 |
10.1063/1.1724828 |
| VEEL5 |
M.E. Jacox, Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules, J. Phys. Chem.Ref. Data, Monograph 3 (1994) (updated data in NIST Chemistry Webbook - http://webbook.nist.gov/chemistry/ |
|
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |