Vibrational levels (cm-1) 
| Mode Number |
Symmetry |
Frequency |
Intensity |
Comment |
Description |
| Fundamental(cm-1) |
Harmonic(cm-1) |
Reference |
(km mol-1) |
unc. |
Reference |
| 1 |
A' |
3022 |
|
1988Mid/Shi:253 |
|
|
|
|
|
| 2 |
A' |
2970 |
|
1988Mid/Shi:253 |
|
|
|
|
|
| 3 |
A' |
2942 |
|
|
|
|
|
|
|
| 4 |
A' |
2940 |
|
|
|
|
|
|
|
| 5 |
A' |
2912 |
|
|
|
|
|
|
|
| 6 |
A' |
1736 |
|
|
|
|
|
|
|
| 7 |
A' |
1485 |
|
|
|
|
|
|
|
| 8 |
A' |
1473 |
|
|
|
|
|
|
|
| 9 |
A' |
1429 |
|
|
|
|
|
|
|
| 10 |
A' |
1399 |
|
|
|
|
|
|
|
| 11 |
A' |
1374 |
|
|
|
|
|
|
|
| 12 |
A' |
1370 |
|
|
|
|
|
|
|
| 13 |
A' |
1262 |
|
|
|
|
|
|
|
| 14 |
A' |
1113 |
|
|
|
|
|
|
|
| 15 |
A' |
1004 |
|
|
|
|
|
|
|
| 16 |
A' |
|
|
|
|
|
|
|
|
| 17 |
A' |
938 |
|
|
|
|
|
|
|
| 18 |
A' |
847 |
|
|
|
|
|
|
|
| 19 |
A' |
635 |
|
|
|
|
|
|
|
| 20 |
A' |
441 |
|
|
|
|
|
|
|
| 21 |
A' |
378 |
|
|
|
|
|
|
|
| 22 |
A' |
160 |
|
|
|
|
|
|
|
| 23 |
A" |
2988 |
|
|
|
|
|
|
|
| 24 |
A" |
2988 |
|
|
|
|
|
|
|
| 25 |
A" |
2985 |
|
|
|
|
|
|
|
| 26 |
A" |
1461 |
|
|
|
|
|
|
|
| 27 |
A" |
1451 |
|
|
|
|
|
|
|
| 28 |
A" |
1267 |
|
|
|
|
|
|
|
| 29 |
A" |
1160 |
|
|
|
|
|
|
|
| 30 |
A" |
1048 |
|
|
|
|
|
|
|
| 31 |
A" |
808 |
|
|
|
|
|
|
|
| 32 |
A" |
602 |
|
|
|
|
|
|
|
| 33 |
A" |
209 |
|
|
|
|
|
|
|
| 34 |
A" |
136 |
|
|
|
|
|
|
|
| 35 |
A" |
|
|
|
|
|
|
|
|
| 36 |
A" |
|
|
|
|
|
|
|
|
vibrational zero-point energy: 24466.5 cm
-1 (from fundamental vibrations)
Calculated vibrational frequencies for
C
4H
8O
2 (Ethyl acetate).
Gas-phase IR spectra can be found in the NIST Chemistry Webbook
here.
Geometric Data
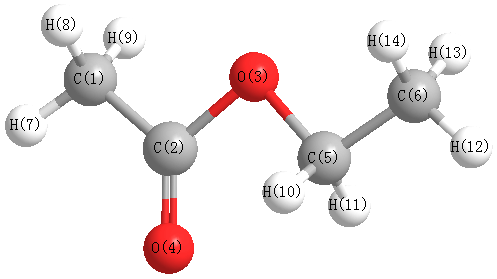
Point Group Cs
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description |
Value |
unc. |
Connectivity |
Reference |
Comment |
| Atom 1 |
Atom 2 |
Atom 3 |
Atom 4 |
| rCH |
1.105 |
|
1 |
7 |
|
|
1998Kuc |
rg |
| rCO |
1.203 |
|
2 |
4 |
|
|
1998Kuc |
rg |
| rCC |
1.508 |
|
1 |
2 |
|
|
1998Kuc |
rg, from =O |
| rCO |
1.345 |
|
2 |
3 |
|
|
1998Kuc |
rg, from =O to O |
| rCO |
1.448 |
|
5 |
6 |
|
|
1998Kuc |
rg, from O |
| rCC |
1.515 |
|
5 |
6 |
|
|
1998Kuc |
rg, from O |
| aCCO |
124.1 |
|
1 |
2 |
4 |
|
1998Kuc |
|
| aOCO |
124 |
|
3 |
2 |
4 |
|
1998Kuc |
|
| aCCO |
111.9 |
|
1 |
2 |
3 |
|
1998Kuc |
dependent |
| aCOC |
115.7 |
|
2 |
3 |
5 |
|
1998Kuc |
|
| aCCO |
108.2 |
|
3 |
5 |
6 |
|
1998Kuc |
|
| aHCC |
107.7 |
|
2 |
1 |
7 |
|
1998Kuc |
from C with =O |
| aHCH |
108.1 |
|
10 |
5 |
11 |
|
1998Kuc |
dependent, C with 2 H |
| aHCO |
108.3 |
|
3 |
5 |
10 |
|
1998Kuc |
dependent, C with 2 H |
| aHCC |
108.1 |
|
5 |
6 |
12 |
|
1998Kuc |
to end C from C with 2 H |
| dHCCO |
0 |
|
4 |
2 |
1 |
7 |
1998Kuc |
!assumed |
| dOCOC |
0 |
|
4 |
2 |
3 |
5 |
1998Kuc |
!assumed |
| dCOCC |
180 |
|
2 |
3 |
5 |
6 |
1998Kuc |
|
| dOCCH |
180 |
|
3 |
5 |
6 |
12 |
1998Kuc |
from middle O |
These cartesians were determined using some assumed coordinate values.
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| O1 |
2.2122 |
0.2727 |
0.0000 |
| C2 |
1.0752 |
-0.1205 |
0.0000 |
| O3 |
0.0000 |
0.6876 |
0.0000 |
| H4 |
0.4586 |
-1.9362 |
0.9117 |
| H5 |
2.0357 |
-2.0141 |
0.0000 |
| H6 |
0.4586 |
-1.9362 |
-0.9117 |
| C7 |
1.0009 |
-1.6266 |
0.0000 |
| H8 |
-1.3528 |
-0.6036 |
-0.9086 |
| H9 |
-1.3528 |
-0.6036 |
0.9086 |
| C10 |
-1.2859 |
0.0218 |
0.0000 |
| H11 |
-2.3515 |
1.6322 |
0.9096 |
| H12 |
-3.3794 |
0.4382 |
0.0000 |
| H13 |
-2.3515 |
1.6322 |
-0.9096 |
| C14 |
-2.4340 |
1.0102 |
0.0000 |
Atom - Atom Distances 
Distances in Å
| |
O1 |
C2 |
O3 |
H4 |
H5 |
H6 |
C7 |
H8 |
H9 |
C10 |
H11 |
H12 |
H13 |
C14 |
| O1 |
|
1.2030 | 2.2507 | 2.9640 | 2.2935 | 2.9640 | 2.2527 | 3.7818 | 3.7818 | 3.5070 | 4.8480 | 5.5940 | 4.8480 | 4.7043 |
| C2 |
1.2030 |
|
1.3450 | 2.1233 | 2.1233 | 2.1233 | 1.5080 | 2.6370 | 2.6370 | 2.3654 | 3.9550 | 4.4895 | 3.9550 | 3.6869 |
| O3 |
2.2507 | 1.3450 |
|
2.8153 | 3.3828 | 2.8153 | 2.5214 | 2.0791 | 2.0791 | 1.4480 | 2.6925 | 3.3886 | 2.6925 | 2.4553 |
| H4 |
2.9640 | 2.1233 | 2.8153 |
|
1.8233 | 1.8233 | 1.1050 | 2.8931 | 2.2488 | 2.7763 | 4.5421 | 4.6043 | 4.8936 | 4.2285 |
| H5 |
2.2935 | 2.1233 | 3.3828 | 1.8233 |
|
1.8233 | 1.1050 | 3.7811 | 3.7811 | 3.8958 | 5.7767 | 5.9445 | 5.7767 | 5.3967 |
| H6 |
2.9640 | 2.1233 | 2.8153 | 1.8233 | 1.8233 |
|
1.1050 | 2.2488 | 2.8931 | 2.7763 | 4.8936 | 4.6043 | 4.5421 | 4.2285 |
| C7 |
2.2527 | 1.5080 | 2.5214 | 1.1050 | 1.1050 | 1.1050 |
|
2.7225 | 2.7225 | 2.8189 | 4.7630 | 4.8426 | 4.7630 | 4.3303 |
| H8 |
3.7818 | 2.6370 | 2.0791 | 2.8931 | 3.7811 | 2.2488 | 2.7225 |
|
1.8171 | 1.1050 | 3.0499 | 2.4532 | 2.4487 | 2.1445 |
| H9 |
3.7818 | 2.6370 | 2.0791 | 2.2488 | 3.7811 | 2.8931 | 2.7225 | 1.8171 |
|
1.1050 | 2.4487 | 2.4532 | 3.0499 | 2.1445 |
| C10 |
3.5070 | 2.3654 | 1.4480 | 2.7763 | 3.8958 | 2.7763 | 2.8189 | 1.1050 | 1.1050 |
|
2.1346 | 2.1346 | 2.1346 | 1.5150 |
| H11 |
4.8480 | 3.9550 | 2.6925 | 4.5421 | 5.7767 | 4.8936 | 4.7630 | 3.0499 | 2.4487 | 2.1346 |
|
1.8192 | 1.8192 | 1.1050 |
| H12 |
5.5940 | 4.4895 | 3.3886 | 4.6043 | 5.9445 | 4.6043 | 4.8426 | 2.4532 | 2.4532 | 2.1346 | 1.8192 |
|
1.8192 | 1.1050 |
| H13 |
4.8480 | 3.9550 | 2.6925 | 4.8936 | 5.7767 | 4.5421 | 4.7630 | 2.4487 | 3.0499 | 2.1346 | 1.8192 | 1.8192 |
|
1.1050 |
| C14 |
4.7043 | 3.6869 | 2.4553 | 4.2285 | 5.3967 | 4.2285 | 4.3303 | 2.1445 | 2.1445 | 1.5150 | 1.1050 | 1.1050 | 1.1050 |
|
Calculated geometries
for C
4H
8O
2 (Ethyl acetate).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| O1 |
C2 |
O3 |
124.000 |
|
O1 |
C2 |
H4 |
123.637 |
| C2 |
O1 |
C7 |
38.398 |
|
C2 |
O1 |
H8 |
14.846 |
| C2 |
O1 |
H9 |
14.846 |
|
C2 |
O3 |
H5 |
16.076 |
| O3 |
C2 |
H4 |
106.357 |
|
O3 |
H5 |
H6 |
56.315 |
| O3 |
H5 |
C10 |
21.497 |
|
O3 |
H5 |
H11 |
16.023 |
| H5 |
H6 |
H12 |
130.093 |
|
H5 |
H6 |
H13 |
124.643 |
| H5 |
H6 |
C14 |
120.908 |
|
H6 |
H5 |
C10 |
40.555 |
| H6 |
H5 |
H11 |
52.761 |
|
C7 |
O1 |
H8 |
45.394 |
| C7 |
O1 |
H9 |
45.394 |
|
H8 |
O1 |
H9 |
27.802 |
| C10 |
H5 |
H11 |
12.213 |
|
H12 |
H6 |
H13 |
22.932 |
| H12 |
H6 |
C14 |
13.525 |
|
H13 |
H6 |
C14 |
13.887 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
8 |
| C-C |
2 |
| C-O |
2 |
| C=O |
1 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
H7 |
| C1 |
H8 |
| C1 |
H9 |
| C2 |
O3 |
| C2 |
O4 |
| O3 |
C5 |
| C5 |
C6 |
| C5 |
H10 |
| C5 |
H11 |
| C6 |
H12 |
| C6 |
H13 |
| C6 |
H14 |












