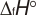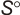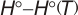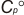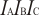Experimental data for C5H10O (2H-Pyran, tetrahydro-)
22 02 02 11 45
| Other names |
|---|
|
2H-Pyran, tetrahydro-; Oxacyclohexane; Oxane; Pentamethylene oxide; Tetrahydro-2H-pyran; Tetrahydropyran; THP;
|
| INChI |
INChIKey |
SMILES |
IUPAC name |
| InChI=1S/C5H10O/c1-2-4-6-5-3-1/h1-5H2 |
DHXVGJBLRPWPCS-UHFFFAOYSA-N |
C1CCCCO1 |
Tetrahydro-2H-pyran |
| State |
Conformation |
| 1A' |
C1 |
Vibrational levels (cm-1) 
| Mode Number |
Symmetry |
Frequency |
Intensity |
Comment |
Description |
| Fundamental(cm-1) |
Harmonic(cm-1) |
Reference |
(km mol-1) |
unc. |
Reference |
Calculated vibrational frequencies for
C
5H
10O (2H-Pyran, tetrahydro-).
Gas-phase IR spectra can be found in the NIST Chemistry Webbook
here.
Geometric Data
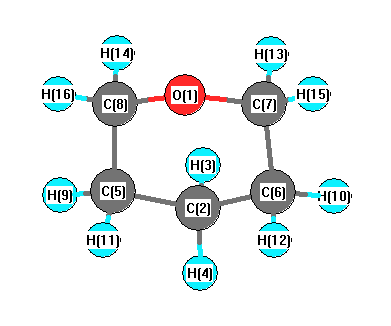
Point Group Cs
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
Atom - Atom Distances 
Distances in Å
Calculated geometries
for C
5H
10O (2H-Pyran, tetrahydro-).
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
10 |
| C-C |
4 |
| C-O |
2 |
Connectivity
| Atom 1 |
Atom 2 |
| O1 |
C7 |
| O1 |
C8 |
| C2 |
H3 |
| C2 |
H4 |
| C2 |
C5 |
| C2 |
C6 |
| C5 |
C8 |
| C5 |
H9 |
| C5 |
H11 |
| C6 |
C7 |
| C6 |
H10 |
| C6 |
H12 |
| C7 |
H13 |
| C7 |
H15 |
| C8 |
H14 |
| C8 |
H16 |
Electronic energy levels (cm-1)
| Energy (cm-1) |
Degeneracy |
reference |
description |
| 0 |
1 |
|
1A' |
Ionization Energies (eV)
| Ionization Energy |
I.E. unc. |
vertical I.E. |
v.I.E. unc. |
reference |
| 9.250 |
0.010 |
9.570 |
|
webbook |
Dipole, Quadrupole and Polarizability
Electric dipole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Dipole (Debye) |
Reference |
comment |
Point Group |
Components |
| x |
y |
z |
total |
dipole |
quadrupole |
| 1 |
1 |
1A' |
C1 |
True |
|
|
|
1.740 |
1974Hel/Hel(II/6) |
μa=1.53± 0.02 μb=0.82± 0.02 |
Cs |
2 |
3 |
Experimental dipole measurement abbreviations: MW microwave; DT Dielectric with Temperature variation; DR Indirect (usually an upper limit); MB Molecular beam
Calculated electric dipole moments for
C
5H
10O (2H-Pyran, tetrahydro-).
Electric quadrupole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Quadrupole (D Å) |
Reference |
comment |
Point Group |
Components |
| xx |
yy |
zz |
dipole |
quadrupole |
| 1 |
1 |
1A' |
C1 |
True |
|
|
|
|
|
Cs |
2 |
3 |
Calculated electric quadrupole moments for
C
5H
10O (2H-Pyran, tetrahydro-).
References
By selecting the following links, you may be leaving NIST webspace.
We have provided these links to other web sites because they may have information that would be of interest to you.
No inferences should be drawn on account of other sites being referenced, or not, from this page.
There may be other web sites that are more appropriate for your purpose.
NIST does not necessarily endorse the views expressed, or concur with the facts presented on these sites.
Further, NIST does not endorse any commercial products that may be mentioned on these sites.
Please address comments about this page to
[email protected].
Got a better number? Please email us at
[email protected]