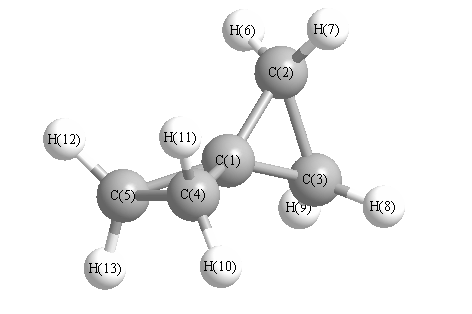
.
| squib |
reference |
DOI |
| 1950Sco/Fin:4664 |
D. W. Scott, H. L. Finke, W. N. Hubbard, J. P. McCullough, M. E. Gross, K. D. Williamson, Guy Waddington, H. M. Huffman "Spiropentane: Heat Capacity, Heats of Fusion and Vaporization, Vapor Pressure, Entropy and Thermodynamic Functions" J. Am. Chem. Soc., 1950, 72 (10), pp 4664–4668 |
10.1021/ja01166a089 |
| 1972Bur/McG:540 |
GR Burns, DG McGavin "Infrared and Raman Spectra fo Spiropentane-H8" Applied Spectroscopy 26(5) 540, 1972 |
10.1366/000370272774351778 |
| 1998Gus/Rui:163 |
M Gussoni, R Rui, G Zerbi "Electronic and relaxation contribution to linear molecular polarizability. An analysis of the experimental values" J. Mol. Struct. 447 (1998) 163-215 |
10.1016/S0022-2860(97)00292-5 |
| 2011Pri/Cou:129-136 |
JE Price, KA Coulterpark, T Masiello, JW Nibler, A Weber, A Maki, TA Blake "High-Resolution infrared spectra of spiropentane, C5H8" J. Mol. Spect. 269 (2011) 129-136 |
10.1016/j.jms.2011.05.011 |
| 2017San/Eri:4923-4929 |
JW Sandwisch, BA Erikson, K Hedberg, JW Niber "Combined Electron-Diffraction and Spectroscopic Determination of the Structure of Spiropentane, C5H8" J. Phys. Chem. A 121, 2017, 4923-4929 |
10.1021/acs.jpca.7b04450 |
| TRC |
Frenkel, M; Marsh, K.N.; Wilhoit, R.C.; Kabo, G.J.; Roganov, G.N.,Thermodynamics of Organic Compounds in the Gas State,Thermodynamics Research Center, College Station, TX, 1994 |
|
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |