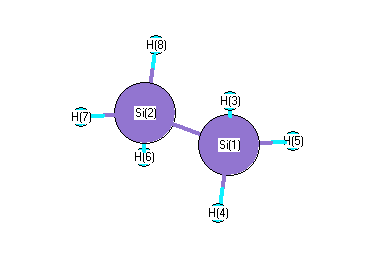
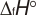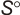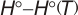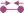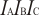.
| squib |
reference |
DOI |
| 1957Bet/Wil:1107 |
GW Bethke, MK Wilson "Vibrational Spectrum of Disilane" J. Chem. Phys. 26(5), 1107, 1957 |
10.1063/1.1743480 |
| 1998Gup/Pra:1607 |
Gupte, Prasad, Ground State Geometries and Vibrational Spectra of Small Hyrdogenated Silicon Clusters Using Nonorthogonal Tight-binding Molecular Dynamics, Inter. J. of Mod. Phys. B, Vol. 12, #15, pgs. 1607-1622 |
10.1142/S0217979298000880 |
| 1998Gus/Rui:163 |
M Gussoni, R Rui, G Zerbi "Electronic and relaxation contribution to linear molecular polarizability. An analysis of the experimental values" J. Mol. Struct. 447 (1998) 163-215 |
10.1016/S0022-2860(97)00292-5 |
| 1998Rap/Ape |
Z Rappoprt, Y Apeloig, "The chemistry of organic silicon compounds Volume 2 Part 1" Chapter 4, Wiley |
10.1021/ja985767u |
| 2003Lat/DiL:2895 |
F Lattanzi, C Di Lauro, V-M Horneman "The nu6, nu8, 3nu4+nu12 infrared system of Si2H6 under high resolution: rotational and torsional analysis" Mol. Phys. 101(18), 2895, 2003 |
10.1080/00268970310001598722 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |