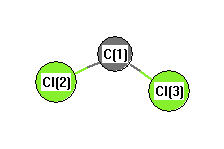
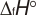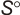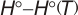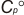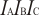.
| squib |
reference |
DOI |
| 2002Dem/Mar:3282 |
J Demaison, L Margules, JML Martin, JE Boggs "Anharmonic force field, structure, and thermochemistry of CF2 and CCl2" Phys. Chem. Chem. Phys. 4(14) 3282-3288, 2002 |
10.1039/b202865d |
| 2002Rie/Tsc:231 |
JC Rienstra-Kiracofe, GS Tschumper, HF Schaefer III, S Nandi, GB Ellison "Atomic and Molecular Electron Affinities: Photoelectron Experiments and Theoretical Computations" Chemical Reviews 2002, 102, 231-282 |
10.1021/cr990044u |
| 2005Tar/Mil:2881 |
G Tarczay, TA Miller, G Czako, AG Csaszar "Accurate ab initio determination of spectroscopic and thermochemical properties of mono- and dichlorocarbenes" Phys. Chem. Chem. Phys. 2005, 7, 4881 and corrections |
10.1039/b506790a |
| JANAF |
Chase, M.W., Jr.; Davies, C.A.; Downey, J.R., Jr.; Frurip, D.J.; McDonald, R.A.; Syverud, A.N., JANAF Thermochemical Tables (Third Edition), J. Phys. Chem. Ref. Data,Suppl. 1, 1985, 14, 1. |
|
| VEEL5 |
M.E. Jacox, Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules, J. Phys. Chem.Ref. Data, Monograph 3 (1994) (updated data in NIST Chemistry Webbook - http://webbook.nist.gov/chemistry/ |
|