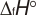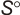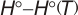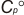.
| squib |
reference |
DOI |
| 1982Jackels:505 |
Jackels, Charles, A theoretical potential energy surface study of several states of the methoxy radical, J. of Chem. Phys., Vol. 76, #1, pgs. 505-515 |
10.1063/1.442752 |
| 1989Liu/Dam:2266 |
Liu, X.; Damo, C.; Lin, T.; Foster, S.; Misra, P.; Yu, L.; Miller, T. "Free Jet-Cooled Laser-Induced Fluorescence Spectrum of Methoxy Radical." Journal of Physical Chemistry. 93, 2266-2275 (1989) |
10.1021/j100343a016 |
| 2002Rie/Tsc:231 |
JC Rienstra-Kiracofe, GS Tschumper, HF Schaefer III, S Nandi, GB Ellison "Atomic and Molecular Electron Affinities: Photoelectron Experiments and Theoretical Computations" Chemical Reviews 2002, 102, 231-282 |
10.1021/cr990044u |
| 2005Rus/Bog:573 |
B Ruscic, JE Boggs, A Burcat, AG Csaszar, J Demaison, R Janoschek, JML Martin, ML Morton, MJ Rossi, JF Stanton, PG Szalay, PR Westmoreland, F Zabel, T Berces "IUPAC Critical Evaluation of Thermochemical Properties of Selected Radicals. Part I" J. Phys. Chem. Ref. Data, Vol. 34, No. 2, 2005, 573 |
10.1063/1.1724828 |
| VEEL5 |
M.E. Jacox, Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules, J. Phys. Chem.Ref. Data, Monograph 3 (1994) (updated data in NIST Chemistry Webbook - http://webbook.nist.gov/chemistry/ |
|
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |