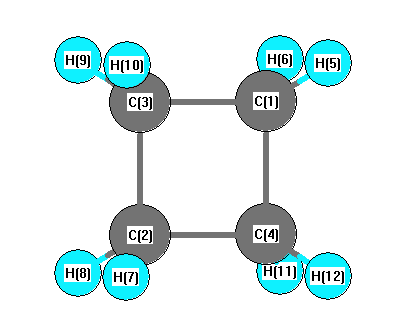
.
| squib |
reference |
DOI |
| 1982Ale/Ant:15 |
VT Aleksanyan BG Antipov "Some Remarks on the Assignment of the Vibrational Spectra of Cyclobutane" J. Mol. Structure 89 (1982) 15-24 |
10.1016/0166-1280(82)80147-4 |
| 1987Ega/Fuk:6018 |
T Egawa, T Fukuyama, S Yamamoto, F Tkabayashi, H Kambara, T Ueda, K Kuchitsu "Molecular structure and puckering potential function of cyclobutane studied by gas electron diffraction and infrared spectroscopy" J. Chem. Phys. 86(11), 6018, 1987 |
10.1063/1.452489 |
| 1998Kuc |
K Kuchitsu(ed) "Structure of Free Polyatomic Molecules - Basic Data" Springer, Berlin, 1998 |
10.1007/978-3-642-45748-7 |
| TRC |
Frenkel, M; Marsh, K.N.; Wilhoit, R.C.; Kabo, G.J.; Roganov, G.N.,Thermodynamics of Organic Compounds in the Gas State,Thermodynamics Research Center, College Station, TX, 1994 |
|
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |