Geometric Data
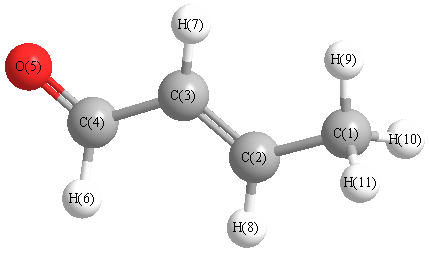
Point Group Cs
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description |
Value |
unc. |
Connectivity |
Reference |
Comment |
| Atom 1 |
Atom 2 |
Atom 3 |
Atom 4 |
| rCC |
1.470 |
|
3 |
4 |
|
|
1976Hellwege(II/7) |
!assumed, from C=O |
| rCC |
1.345 |
|
2 |
3 |
|
|
1976Hellwege(II/7) |
!assumed |
| rCC |
1.501 |
|
1 |
2 |
|
|
1976Hellwege(II/7) |
!assumed, to end |
| rCO |
1.219 |
|
4 |
5 |
|
|
1976Hellwege(II/7) |
!assumed |
| rCH |
1.108 |
|
4 |
6 |
|
|
1976Hellwege(II/7) |
!assumed, C with =O |
| rCH |
1.086 |
|
2 |
8 |
|
|
1976Hellwege(II/7) |
!assumed, C with =C away from O |
| rCH |
1.084 |
|
3 |
7 |
|
|
1976Hellwege(II/7) |
!assumed, C with =C towards O |
| rCH |
1.090 |
|
1 |
9 |
|
|
1976Hellwege(II/7) |
!assumed, end C |
| aCCC |
119.83 |
|
2 |
3 |
4 |
|
1976Hellwege(II/7) |
!assumed, towards =O |
| aCCO |
123.28 |
|
3 |
4 |
5 |
|
1976Hellwege(II/7) |
!assumed |
| aHCC |
115.1 |
|
3 |
4 |
6 |
|
1976Hellwege(II/7) |
!assumed, middle C has =O |
| aHCH |
109.47 |
|
9 |
1 |
10 |
|
1976Hellwege(II/7) |
!assumed, end C |
| aHCC |
109.47 |
|
2 |
1 |
9 |
|
1976Hellwege(II/7) |
!assumed, towards end C |
| aHCC |
122.83 |
|
2 |
3 |
7 |
|
1976Hellwege(II/7) |
!assumed |
| aCCC |
125.64 |
|
2 |
3 |
4 |
|
1976Hellwege(II/7) |
|
| aHCC |
116.09 |
|
3 |
4 |
6 |
|
1976Hellwege(II/7) |
|
| dHCCH |
120.5 |
|
9 |
1 |
2 |
10 |
|
from HF/6-31G* |
These cartesians were determined using some assumed coordinate values.
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
2.3582 |
-0.5752 |
0.0000 |
| C2 |
0.8612 |
-0.6845 |
0.0000 |
| C3 |
0.0000 |
0.3486 |
0.0000 |
| C4 |
-1.4477 |
0.0937 |
0.0000 |
| O5 |
-2.2832 |
0.9813 |
0.0000 |
| H6 |
-1.7366 |
-0.9760 |
0.0000 |
| H7 |
0.3239 |
1.3851 |
0.0000 |
| H8 |
0.5379 |
-1.7192 |
0.0000 |
| H9 |
2.7651 |
0.4360 |
0.0000 |
| H10 |
2.8725 |
-1.0359 |
0.8435 |
| H11 |
2.8725 |
-1.0359 |
-0.8435 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
C2 |
C3 |
C4 |
O5 |
H6 |
H7 |
H8 |
H9 |
H10 |
H11 |
| C1 |
|
1.5010 | 2.5327 | 3.8643 | 4.8955 | 4.1144 | 2.8252 | 2.1500 | 1.0900 | 1.0900 | 1.0900 |
| C2 |
1.5010 |
|
1.3450 | 2.4366 | 3.5585 | 2.6142 | 2.1383 | 1.0840 | 2.2091 | 2.2091 | 2.2091 |
| C3 |
2.5327 | 1.3450 |
|
1.4700 | 2.3693 | 2.1841 | 1.0860 | 2.1366 | 2.7664 | 3.2984 | 3.2984 |
| C4 |
3.8643 | 2.4366 | 1.4700 |
|
1.2190 | 1.1080 | 2.1924 | 2.6887 | 4.2267 | 4.5444 | 4.5444 |
| O5 |
4.8955 | 3.5585 | 2.3693 | 1.2190 |
|
2.0322 | 2.6382 | 3.9053 | 5.0776 | 5.6002 | 5.6002 |
| H6 |
4.1144 | 2.6142 | 2.1841 | 1.1080 | 2.0322 |
|
3.1338 | 2.3929 | 4.7179 | 4.6861 | 4.6861 |
| H7 |
2.8252 | 2.1383 | 1.0860 | 2.1924 | 2.6382 | 3.1338 |
|
3.1117 | 2.6191 | 3.6150 | 3.6150 |
| H8 |
2.1500 | 1.0840 | 2.1366 | 2.6887 | 3.9053 | 2.3929 | 3.1117 |
|
3.0992 | 2.5747 | 2.5747 |
| H9 |
1.0900 | 2.2091 | 2.7664 | 4.2267 | 5.0776 | 4.7179 | 2.6191 | 3.0992 |
|
1.6998 | 1.6998 |
| H10 |
1.0900 | 2.2091 | 3.2984 | 4.5444 | 5.6002 | 4.6861 | 3.6150 | 2.5747 | 1.6998 |
|
1.6870 |
| H11 |
1.0900 | 2.2091 | 3.2984 | 4.5444 | 5.6002 | 4.6861 | 3.6150 | 2.5747 | 1.6998 | 1.6870 |
|
Calculated geometries
for CHOCHCHCH
3 (2-Butenal).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
C3 |
125.640 |
|
C1 |
C2 |
H8 |
111.530 |
| C2 |
C1 |
H9 |
116.090 |
|
C2 |
C1 |
H10 |
116.090 |
| C2 |
C1 |
H11 |
116.090 |
|
C2 |
C3 |
C4 |
119.830 |
| C2 |
C3 |
H7 |
122.830 |
|
C3 |
C2 |
H8 |
122.830 |
| C3 |
C4 |
O5 |
123.280 |
|
C3 |
C4 |
H6 |
115.100 |
| C4 |
C3 |
H7 |
117.340 |
|
O5 |
C4 |
H6 |
121.620 |
| H9 |
C1 |
H10 |
102.472 |
|
H9 |
C1 |
H11 |
102.472 |
| H10 |
C1 |
H11 |
101.399 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
6 |
| C-C |
2 |
| C=C |
1 |
| C=O |
1 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
H9 |
| C1 |
H10 |
| C1 |
H11 |
| C2 |
C3 |
| C2 |
H8 |
| C3 |
C4 |
| C3 |
H7 |
| C4 |
O5 |
| C4 |
H6 |












