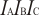Experimental data for CH3CF3 (Ethane, 1,1,1-trifluoro-)
22 02 02 11 45
Enthalpy of formation (Hfg),
Entropy,
Integrated heat capacity (0 K to 298.15 K) (HH),
Heat Capacity (Cp)
| Property |
Value |
Uncertainty |
units |
Reference |
Comment |
Information can also be found for this species in the
NIST Chemistry Webbook
Vibrational levels (cm-1) 
| Mode Number |
Symmetry |
Frequency |
Intensity |
Comment |
Description |
| Fundamental(cm-1) |
Harmonic(cm-1) |
Reference |
(km mol-1) |
unc. |
Reference |
vibrational zero-point energy: cm
-1 (from fundamental vibrations)
Calculated vibrational frequencies for
CH
3CF
3 (Ethane, 1,1,1-trifluoro-).
Geometric Data

Point Group
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description |
Value |
unc. |
Connectivity |
Reference |
Comment |
| Atom 1 |
Atom 2 |
Atom 3 |
Atom 4 |
Cartesians
Atom - Atom Distances 
Distances in Å
Calculated geometries
for CH
3CF
3 (Ethane, 1,1,1-trifluoro-).
Electronic energy levels (cm-1)
| Energy (cm-1) |
Degeneracy |
reference |
description |
Dipole, Quadrupole and Polarizability
Electric dipole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Dipole (Debye) |
Reference |
comment |
Point Group |
Components |
| x |
y |
z |
total |
dipole |
quadrupole |
Experimental dipole measurement abbreviations: MW microwave; DT Dielectric with Temperature variation; DR Indirect (usually an upper limit); MB Molecular beam
Calculated electric dipole moments for
CH
3CF
3 (Ethane, 1,1,1-trifluoro-).
Electric quadrupole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Quadrupole (D Å) |
Reference |
comment |
Point Group |
Components |
| xx |
yy |
zz |
dipole |
quadrupole |
Calculated electric quadrupole moments for
CH
3CF
3 (Ethane, 1,1,1-trifluoro-).