Geometric Data
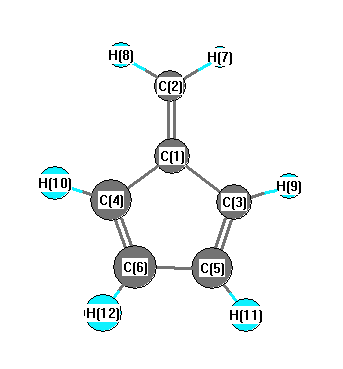
Point Group C2v
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
0.0000 |
0.0000 |
0.7693 |
| C2 |
0.0000 |
0.0000 |
2.1178 |
| C3 |
0.0000 |
1.1788 |
-0.1090 |
| C4 |
0.0000 |
-1.1788 |
-0.1090 |
| C5 |
0.0000 |
0.7382 |
-1.3901 |
| C6 |
0.0000 |
-0.7382 |
-1.3901 |
| H7 |
0.0000 |
0.9624 |
2.7037 |
| H8 |
0.0000 |
-0.9624 |
2.7037 |
| H9 |
0.0000 |
2.2000 |
0.2358 |
| H10 |
0.0000 |
-2.2000 |
0.2358 |
| H11 |
0.0000 |
1.3517 |
-2.2785 |
| H12 |
0.0000 |
-1.3517 |
-2.2785 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
H7 |
H8 |
H9 |
H10 |
H11 |
H12 |
| C1 |
|
1.3485 | 1.4700 | 1.4700 | 2.2821 | 2.2821 | 2.1606 | 2.1606 | 2.2638 | 2.2638 | 3.3341 | 3.3341 |
| C2 |
1.3485 |
|
2.5196 | 2.5196 | 3.5847 | 3.5847 | 1.1267 | 1.1267 | 2.8952 | 2.8952 | 4.5994 | 4.5994 |
| C3 |
1.4700 | 2.5196 |
|
2.3576 | 1.3547 | 2.3057 | 2.8210 | 3.5350 | 1.0779 | 3.3964 | 2.1764 | 3.3332 |
| C4 |
1.4700 | 2.5196 | 2.3576 |
|
2.3057 | 1.3547 | 3.5350 | 2.8210 | 3.3964 | 1.0779 | 3.3332 | 2.1764 |
| C5 |
2.2821 | 3.5847 | 1.3547 | 2.3057 |
|
1.4764 | 4.0999 | 4.4330 | 2.1864 | 3.3581 | 1.0797 | 2.2709 |
| C6 |
2.2821 | 3.5847 | 2.3057 | 1.3547 | 1.4764 |
|
4.4330 | 4.0999 | 3.3581 | 2.1864 | 2.2709 | 1.0797 |
| H7 |
2.1606 | 1.1267 | 2.8210 | 3.5350 | 4.0999 | 4.4330 |
|
1.9248 | 2.7608 | 4.0114 | 4.9974 | 5.4934 |
| H8 |
2.1606 | 1.1267 | 3.5350 | 2.8210 | 4.4330 | 4.0999 | 1.9248 |
|
4.0114 | 2.7608 | 5.4934 | 4.9974 |
| H9 |
2.2638 | 2.8952 | 1.0779 | 3.3964 | 2.1864 | 3.3581 | 2.7608 | 4.0114 |
|
4.4000 | 2.6535 | 4.3516 |
| H10 |
2.2638 | 2.8952 | 3.3964 | 1.0779 | 3.3581 | 2.1864 | 4.0114 | 2.7608 | 4.4000 |
|
4.3516 | 2.6535 |
| H11 |
3.3341 | 4.5994 | 2.1764 | 3.3332 | 1.0797 | 2.2709 | 4.9974 | 5.4934 | 2.6535 | 4.3516 |
|
2.7035 |
| H12 |
3.3341 | 4.5994 | 3.3332 | 2.1764 | 2.2709 | 1.0797 | 5.4934 | 4.9974 | 4.3516 | 2.6535 | 2.7035 |
|
Calculated geometries
for C
6H
6 (Fulvene).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
H7 |
121.333 |
|
C1 |
C2 |
H8 |
121.333 |
| C1 |
C3 |
C5 |
107.710 |
|
C1 |
C3 |
H9 |
124.654 |
| C1 |
C4 |
C6 |
107.710 |
|
C1 |
C4 |
H10 |
124.654 |
| C2 |
C1 |
C3 |
126.689 |
|
C2 |
C1 |
C4 |
126.689 |
| C3 |
C1 |
C4 |
106.622 |
|
C3 |
C5 |
C6 |
108.979 |
| C3 |
C5 |
H11 |
126.392 |
|
C4 |
C6 |
C5 |
108.979 |
| C4 |
C6 |
H12 |
126.392 |
|
C5 |
C3 |
H9 |
127.636 |
| C5 |
C6 |
H12 |
124.629 |
|
C6 |
C4 |
H10 |
127.636 |
| C6 |
C5 |
H11 |
124.629 |
|
H7 |
C2 |
H8 |
117.335 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
6 |
| C-C |
3 |
| C=C |
3 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
C3 |
| C1 |
C4 |
| C2 |
H7 |
| C2 |
H8 |
| C3 |
C5 |
| C3 |
H9 |
| C4 |
C6 |
| C4 |
H10 |
| C5 |
C6 |
| C5 |
H11 |
| C6 |
H12 |










