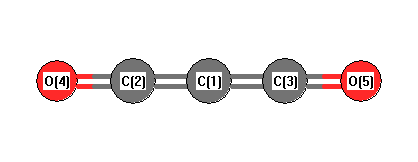
.
| squib |
reference |
DOI |
| 1966Herzberg |
Herzberg, G., Electronic spectra and electronic structure of polyatomic molecules,Van Nostrand,New York, 1966 |
|
| 2001ELL/DRE:73 |
A. Ellern, T Drews, K. Seppelt "The structure of carbon suboxide, C3O2, in the solid state" Z. Anorg. Allg. Chem. 2001, 627, 73-76 |
10.1002/1521-3749(200101)627:1<73::AID-ZAAC73>3.0.CO;2-A |
| Gurvich |
Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub. Co., New York, 1989 |
|
| NSRDS-NBS10 |
R. D. Nelson Jr., D. R. Lide, A. A. Maryott "Selected Values of electric dipole moments for molecules in the gas phase" NSRDS-NBS10, 1967 |
10.6028/NBS.NSRDS.10 |
| Shim |
Shimanouchi, T. , Tables of Molecular Vibrational Frequencies, Consolidated Volu |
10.6028/NBS.NSRDS.39 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |