Geometric Data
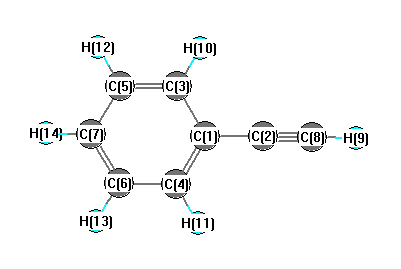
Point Group C2v
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
0.0000 |
0.0000 |
-0.5514 |
| C2 |
0.0000 |
0.0000 |
-1.9996 |
| C3 |
0.0000 |
-1.2067 |
0.1344 |
| C4 |
0.0000 |
1.2067 |
0.1344 |
| C5 |
0.0000 |
-1.2105 |
1.5299 |
| C6 |
0.0000 |
1.2105 |
1.5299 |
| C7 |
0.0000 |
0.0000 |
2.2310 |
| C8 |
0.0000 |
0.0000 |
-3.2079 |
| H9 |
0.0000 |
0.0000 |
-4.2631 |
| H10 |
0.0000 |
-2.1396 |
-0.4102 |
| H11 |
0.0000 |
2.1396 |
-0.0410 |
| H12 |
0.0000 |
-2.1467 |
2.0726 |
| H13 |
0.0000 |
2.1467 |
2.0726 |
| H14 |
0.0000 |
0.0000 |
3.3106 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
C7 |
C8 |
H9 |
H10 |
H11 |
H12 |
H13 |
H14 |
| C1 |
|
1.4482 | 1.3880 | 1.3880 | 2.4077 | 2.4077 | 2.7824 | 2.6565 | 3.7117 | 2.1443 | 2.1996 | 3.3902 | 3.3902 | 3.8620 |
| C2 |
1.4482 |
|
2.4515 | 2.4515 | 3.7313 | 3.7313 | 4.2306 | 1.2083 | 2.2635 | 2.6653 | 2.9007 | 4.6034 | 4.6034 | 5.3102 |
| C3 |
1.3880 | 2.4515 |
|
2.4134 | 1.3955 | 2.7911 | 2.4191 | 3.5535 | 4.5601 | 1.0802 | 3.3509 | 2.1541 | 3.8732 | 3.3977 |
| C4 |
1.3880 | 2.4515 | 2.4134 |
|
2.7911 | 1.3955 | 2.4191 | 3.5535 | 4.5601 | 3.3903 | 0.9492 | 3.8732 | 2.1541 | 3.3977 |
| C5 |
2.4077 | 3.7313 | 1.3955 | 2.7911 |
|
2.4210 | 1.3989 | 4.8900 | 5.9181 | 2.1511 | 3.7001 | 1.0821 | 3.4008 | 2.1532 |
| C6 |
2.4077 | 3.7313 | 2.7911 | 1.3955 | 2.4210 |
|
1.3989 | 4.8900 | 5.9181 | 3.8713 | 1.8251 | 3.4008 | 1.0821 | 2.1532 |
| C7 |
2.7824 | 4.2306 | 2.4191 | 2.4191 | 1.3989 | 1.3989 |
|
5.4389 | 6.4941 | 3.3991 | 3.1209 | 2.1525 | 2.1525 | 1.0796 |
| C8 |
2.6565 | 1.2083 | 3.5535 | 3.5535 | 4.8900 | 4.8900 | 5.4389 |
|
1.0552 | 3.5221 | 3.8219 | 5.7002 | 5.7002 | 6.5185 |
| H9 |
3.7117 | 2.2635 | 4.5601 | 4.5601 | 5.9181 | 5.9181 | 6.4941 | 1.0552 |
|
4.4071 | 4.7333 | 6.6895 | 6.6895 | 7.5737 |
| H10 |
2.1443 | 2.6653 | 1.0802 | 3.3903 | 2.1511 | 3.8713 | 3.3991 | 3.5221 | 4.4071 |
|
4.2951 | 2.4828 | 4.9534 | 4.2921 |
| H11 |
2.1996 | 2.9007 | 3.3509 | 0.9492 | 3.7001 | 1.8251 | 3.1209 | 3.8219 | 4.7333 | 4.2951 |
|
4.7791 | 2.1136 | 3.9763 |
| H12 |
3.3902 | 4.6034 | 2.1541 | 3.8732 | 1.0821 | 3.4008 | 2.1525 | 5.7002 | 6.6895 | 2.4828 | 4.7791 |
|
4.2934 | 2.4781 |
| H13 |
3.3902 | 4.6034 | 3.8732 | 2.1541 | 3.4008 | 1.0821 | 2.1525 | 5.7002 | 6.6895 | 4.9534 | 2.1136 | 4.2934 |
|
2.4781 |
| H14 |
3.8620 | 5.3102 | 3.3977 | 3.3977 | 2.1532 | 2.1532 | 1.0796 | 6.5185 | 7.5737 | 4.2921 | 3.9763 | 2.4781 | 2.4781 |
|
Calculated geometries
for C
6H
5CCH (phenylacetylene).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
C8 |
180.000 |
|
C1 |
C3 |
C5 |
119.767 |
| C1 |
C3 |
H10 |
120.114 |
|
C1 |
C4 |
C6 |
119.767 |
| C1 |
C4 |
H11 |
139.740 |
|
C2 |
C1 |
C3 |
119.611 |
| C2 |
C1 |
C4 |
119.611 |
|
C2 |
C8 |
H9 |
180.000 |
| C3 |
C1 |
C4 |
120.779 |
|
C3 |
C5 |
C7 |
119.923 |
| C3 |
C5 |
H12 |
120.256 |
|
C4 |
C6 |
C7 |
119.923 |
| C4 |
C6 |
H13 |
120.256 |
|
C5 |
C3 |
H10 |
120.119 |
| C5 |
C7 |
C6 |
119.843 |
|
C5 |
C7 |
H14 |
120.079 |
| C6 |
C4 |
H11 |
100.493 |
|
C6 |
C7 |
H14 |
120.079 |
| C7 |
C5 |
H12 |
119.821 |
|
C7 |
C6 |
H13 |
119.821 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| C:C |
6 |
| C-C |
1 |
| C#C |
1 |
| H-C |
6 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
C3 |
| C1 |
C4 |
| C2 |
C8 |
| C3 |
C5 |
| C3 |
H10 |
| C4 |
C6 |
| C4 |
H11 |
| C5 |
C7 |
| C5 |
H12 |
| C6 |
C7 |
| C6 |
H13 |
| C7 |
H14 |
| C8 |
H9 |









