Geometric Data
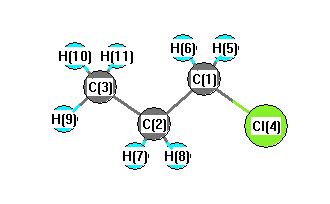
Point Group C1
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
0.0000 |
0.5937 |
0.0000 |
| C2 |
-0.8957 |
-0.6405 |
0.0000 |
| C3 |
-2.3710 |
-0.2543 |
0.0000 |
| Cl4 |
1.7375 |
0.1388 |
0.0000 |
| H5 |
-0.1639 |
1.2199 |
-0.9054 |
| H6 |
-0.1639 |
1.2199 |
0.9054 |
| H7 |
-0.6638 |
-1.2607 |
0.8947 |
| H8 |
-0.6638 |
-1.2607 |
-0.8947 |
| H9 |
-3.0133 |
-1.1633 |
0.0000 |
| H10 |
-2.6338 |
0.3458 |
0.8998 |
| H11 |
-2.6338 |
0.3458 |
-0.8998 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
C2 |
C3 |
Cl4 |
H5 |
H6 |
H7 |
H8 |
H9 |
H10 |
H11 |
| C1 |
|
1.5250 | 2.5181 | 1.7960 | 1.1130 | 1.1130 | 2.1632 | 2.1632 | 3.4881 | 2.7942 | 2.7942 |
| C2 |
1.5250 |
|
1.5250 | 2.7461 | 2.1947 | 2.1947 | 1.1130 | 1.1130 | 2.1812 | 2.1916 | 2.1916 |
| C3 |
2.5181 | 1.5250 |
|
4.1272 | 2.8044 | 2.8044 | 2.1743 | 2.1743 | 1.1130 | 1.1130 | 1.1130 |
| Cl4 |
1.7960 | 2.7461 | 4.1272 |
|
2.3672 | 2.3672 | 2.9198 | 2.9198 | 4.9260 | 4.4677 | 4.4677 |
| H5 |
1.1130 | 2.1947 | 2.8044 | 2.3672 |
|
1.8107 | 3.1054 | 2.5305 | 3.8235 | 3.1817 | 2.6200 |
| H6 |
1.1130 | 2.1947 | 2.8044 | 2.3672 | 1.8107 |
|
2.5305 | 3.1054 | 3.8235 | 2.6200 | 3.1817 |
| H7 |
2.1632 | 1.1130 | 2.1743 | 2.9198 | 3.1054 | 2.5305 |
|
1.7893 | 2.5160 | 2.5420 | 3.1115 |
| H8 |
2.1632 | 1.1130 | 2.1743 | 2.9198 | 2.5305 | 3.1054 | 1.7893 |
|
2.5160 | 3.1115 | 2.5420 |
| H9 |
3.4881 | 2.1812 | 1.1130 | 4.9260 | 3.8235 | 3.8235 | 2.5160 | 2.5160 |
|
1.7975 | 1.7975 |
| H10 |
2.7942 | 2.1916 | 1.1130 | 4.4677 | 3.1817 | 2.6200 | 2.5420 | 3.1115 | 1.7975 |
|
1.7995 |
| H11 |
2.7942 | 2.1916 | 1.1130 | 4.4677 | 2.6200 | 3.1817 | 3.1115 | 2.5420 | 1.7975 | 1.7995 |
|
Calculated geometries
for CH
2ClCH
2CH
3 (Propane, 1-chloro-).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
C3 |
111.300 |
|
C1 |
C2 |
H7 |
109.179 |
| C1 |
C2 |
H8 |
109.179 |
|
C2 |
C1 |
Cl4 |
111.300 |
| C2 |
C1 |
H5 |
111.648 |
|
C2 |
C1 |
H6 |
111.648 |
| C2 |
C3 |
H9 |
110.578 |
|
C2 |
C3 |
H10 |
111.404 |
| C2 |
C3 |
H11 |
111.404 |
|
C3 |
C2 |
H7 |
110.042 |
| C3 |
C2 |
H8 |
110.042 |
|
Cl4 |
C1 |
H5 |
106.556 |
| Cl4 |
C1 |
H6 |
106.556 |
|
H5 |
C1 |
H6 |
108.869 |
| H7 |
C2 |
H8 |
106.995 |
|
H9 |
C3 |
H10 |
107.704 |
| H9 |
C3 |
H11 |
107.704 |
|
H10 |
C3 |
H11 |
107.881 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
7 |
| C-C |
2 |
| C-Cl |
1 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
Cl4 |
| C1 |
H5 |
| C1 |
H6 |
| C2 |
C3 |
| C2 |
H7 |
| C2 |
H8 |
| C3 |
H9 |
| C3 |
H10 |
| C3 |
H11 |












