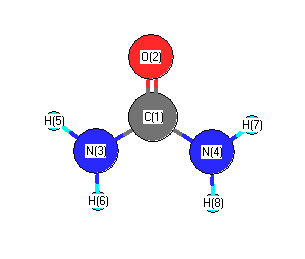
.
| squib |
reference |
DOI |
| 1975Bro/God:445 |
RD Brown, PD Godfrey, J Storey "The Microwave Spectrum of Urea" J. Molec. Spect. 58, 445-450 (1975) |
10.1016/0022-2852(75)90224-6 |
| 1993Vij/Sat:245 |
Vijay and Sathyanarayana. Ab initio study of the force field, geometry and vibrational assignment of urea. J. Mol. Struct. Vol. 295. pgs. 245-258. |
10.1016/0022-2860(93)85024-O |
| 1997God/Bro:405 |
Godfrey, P., Brown, R., Hunter, A., The Shape of Urea, J. of Mol. Struct., V413-414,(1997), pgs. 405-414 |
10.1016/S0022-2860(97)00176-2 |
| TRC |
Frenkel, M; Marsh, K.N.; Wilhoit, R.C.; Kabo, G.J.; Roganov, G.N.,Thermodynamics of Organic Compounds in the Gas State,Thermodynamics Research Center, College Station, TX, 1994 |
|
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |