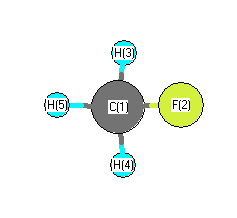
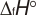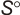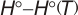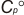.
| squib |
reference |
DOI |
| 1971Fly/Ben:225 |
WH Flygare, RC Benson "The molecular Zeeman effect in diamagnetic molecules and the determination of molecular magnetic moments (g values), magnetic susceptibilities, and molecular quadrupole moments" Mol. Phys. 1971, 20 (2), 225-250 |
10.1080/00268977100100221 |
| 1974Hel/Hel(II/6) |
Hellwege, KH and AM Hellwege (eds.). Landolt-Bornstein: Group II: Volume 6 Molecular Constants from Microwave, Molecular Beam, and Electron Spin Resonance Spectroscopy Springer-Verlag. Berlin. 1974. |
10.1007/b19951 |
| 1993Pap/Hsu:33 |
Papousek, D.; Hsu, Y.C.; Chen, H.S.; Pracna, P.; Klee, S.; Winnewisser, M. "Far Infrared Specturm and Ground State Parameters of 12CH3F." Journal of Molecular Spectroscopy. 159, 33-41 (1993) |
10.1006/jmsp.1993.1102 |
| 1997Oln/Can:59 |
TN Olney, NM Cann, G Cooper, CE Brion, Absolute scale determination for photoabsorption spectra and the calculation of molecular properties using dipole sum-rules, Chem. Phys. 223 (1997) 59-98 |
10.1016/S0301-0104(97)00145-6 |
| 1999Dem/Bre:129 |
Demaison, Breidung, Thiel, Papousek, The Equilibrium Structure of Methyl Fluoride, Structural Chemistry, Vol. 10, #2, pgs. 129-133 |
10.1023/A:1022085314343 |
| Gurvich |
Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub. Co., New York, 1989 |
|
| Shim |
Shimanouchi, T. , Tables of Molecular Vibrational Frequencies, Consolidated Volu |
10.6028/NBS.NSRDS.39 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |