Geometric Data
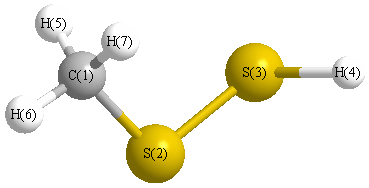
Point Group C1
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
-1.6463 |
0.6787 |
-0.0033 |
| S2 |
-0.4606 |
-0.7060 |
0.0127 |
| S3 |
1.3362 |
0.2507 |
-0.0867 |
| H4 |
1.5393 |
0.4174 |
1.2263 |
| H5 |
-1.4978 |
1.2802 |
-0.8965 |
| H6 |
-2.6411 |
0.2362 |
-0.0211 |
| H7 |
-1.5319 |
1.2789 |
0.8957 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
S2 |
S3 |
H4 |
H5 |
H6 |
H7 |
| C1 |
|
1.8230 | 3.0141 | 3.4246 | 1.0870 | 1.0890 | 1.0870 |
| S2 |
1.8230 |
|
2.0380 | 2.5950 | 2.4181 | 2.3756 | 2.4222 |
| S3 |
3.0141 | 2.0380 |
|
1.3390 | 3.1220 | 3.9779 | 3.2012 |
| H4 |
3.4246 | 2.5950 | 1.3390 |
|
3.8045 | 4.3663 | 3.2067 |
| H5 |
1.0870 | 2.4181 | 3.1220 | 3.8045 |
|
1.7786 | 1.7925 |
| H6 |
1.0890 | 2.3756 | 3.9779 | 4.3663 | 1.7786 |
|
1.7771 |
| H7 |
1.0870 | 2.4222 | 3.2012 | 3.2067 | 1.7925 | 1.7771 |
|
Calculated geometries
for CH
3SSH (Hydrogen methyl disulfide).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
S2 |
S3 |
102.500 |
|
S2 |
C1 |
H5 |
109.800 |
| S2 |
C1 |
H6 |
106.600 |
|
S2 |
C1 |
H7 |
110.100 |
| S2 |
S3 |
H4 |
98.300 |
|
H5 |
C1 |
H6 |
109.647 |
| H5 |
C1 |
H7 |
111.078 |
|
H6 |
C1 |
H7 |
109.512 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
3 |
| H-S |
1 |
| C-S |
1 |
| S-S |
1 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
S2 |
| C1 |
H5 |
| C1 |
H6 |
| C1 |
H7 |
| S2 |
S3 |
| S3 |
H4 |
Dipole, Quadrupole and Polarizability
Electric dipole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Dipole (Debye) |
Reference |
comment |
Point Group |
Components |
| x |
y |
z |
total |
dipole |
quadrupole |
| 1 |
1 |
1A |
C1 |
True |
1.082 |
1.217 |
0.757 |
1.796 |
1986Tyb/Ha:353 |
a=x, b=y, c=z |
C1 |
3 |
5 |
Experimental dipole measurement abbreviations: MW microwave; DT Dielectric with Temperature variation; DR Indirect (usually an upper limit); MB Molecular beam
Calculated electric dipole moments for
CH
3SSH (Hydrogen methyl disulfide).
Electric quadrupole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Quadrupole (D Å) |
Reference |
comment |
Point Group |
Components |
| xx |
yy |
zz |
dipole |
quadrupole |
| 1 |
1 |
1A |
C1 |
True |
|
|
|
|
|
C1 |
3 |
5 |
Calculated electric quadrupole moments for
CH
3SSH (Hydrogen methyl disulfide).







