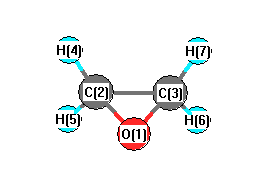
.
| squib |
reference |
DOI |
| 1966Herzberg |
Herzberg, G., Electronic spectra and electronic structure of polyatomic molecules,Van Nostrand,New York, 1966 |
|
| 1971Fly/Ben:225 |
WH Flygare, RC Benson "The molecular Zeeman effect in diamagnetic molecules and the determination of molecular magnetic moments (g values), magnetic susceptibilities, and molecular quadrupole moments" Mol. Phys. 1971, 20 (2), 225-250 |
10.1080/00268977100100221 |
| 1988Cou/Job:213 |
C Coulombeau, H Jobic "Neutron Inelastic Spectroscopy of Ethylene Oxide and Partial Reassignment of the Vibrational Frequencies" J. Molecular Structure 176 (1988) 213-222 |
10.1016/0022-2860(88)80242-4 |
| 1995Kuchitsu(II/23) |
Kuchitsu (ed.), Landolt-Bornstein: Group II: Molecules and Radicals Volume 23: Structure Data for Free Polyatomic Molecules. Springer. Berlin. 1995 |
|
| 1998Gus/Rui:163 |
M Gussoni, R Rui, G Zerbi "Electronic and relaxation contribution to linear molecular polarizability. An analysis of the experimental values" J. Mol. Struct. 447 (1998) 163-215 |
10.1016/S0022-2860(97)00292-5 |
| 1998Hun/Lia:413 |
EPL Hunter, SG Lias "Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update" J. Phys. Chem. Ref. Data Vol. 27, No. 3, pp. 413-656 (1998) |
10.1063/1.556018 |
| JANAF |
Chase, M.W., Jr.; Davies, C.A.; Downey, J.R., Jr.; Frurip, D.J.; McDonald, R.A.; Syverud, A.N., JANAF Thermochemical Tables (Third Edition), J. Phys. Chem. Ref. Data,Suppl. 1, 1985, 14, 1. |
|
| NSRDS-NBS10 |
R. D. Nelson Jr., D. R. Lide, A. A. Maryott "Selected Values of electric dipole moments for molecules in the gas phase" NSRDS-NBS10, 1967 |
10.6028/NBS.NSRDS.10 |
| Shim |
Shimanouchi, T. , Tables of Molecular Vibrational Frequencies, Consolidated Volu |
10.6028/NBS.NSRDS.39 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |