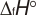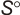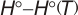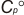.
| squib |
reference |
DOI |
| 1974Hel/Hel(II/6) |
Hellwege, KH and AM Hellwege (eds.). Landolt-Bornstein: Group II: Volume 6 Molecular Constants from Microwave, Molecular Beam, and Electron Spin Resonance Spectroscopy Springer-Verlag. Berlin. 1974. |
10.1007/b19951 |
| 1979HUB/HER |
Huber, K.P.; Herzberg, G., Molecular Spectra and Molecular Structure. IV. Constants of Diatomic Molecules, Van Nostrand Reinhold Co., 1979 |
10.1007/978-1-4757-0961-2 |
| 1998Gus/Rui:163 |
M Gussoni, R Rui, G Zerbi "Electronic and relaxation contribution to linear molecular polarizability. An analysis of the experimental values" J. Mol. Struct. 447 (1998) 163-215 |
10.1016/S0022-2860(97)00292-5 |
| 1999LeR:189 |
RJ Le Roy "Improved Parameterization from Combined Isotopomer Analysis of Diatomic Spectra and Its Application to HF and DF" J. Mol. Spect. 194, 189-196 (1999) |
10.1006/jmsp.1998.7786 |
| 2007Iri:389 |
KK Irikura "Experimental Vibrational Zero-Point Energies: Diatomic Molecules" J. Phys. Chem. Ref. Data 36(2), 389, 2007 |
10.1063/1.2436891 |
| CODATA |
Cox, J.D.; Wagman, D.D.; Medvedev, V.A.CODATA Key Values for Thermodynamics. Hemisphere, New York, 1989 |
10.1002/bbpc.19900940121 |
| Gurvich |
Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub. Co., New York, 1989 |
|
| NISTdiatomic |
NIST Diatomic Spectral Database (www.physics.nist.gov/PhysRefData/MolSpec/Diatomic/index.html) |
10.18434/T4T59X |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |