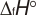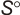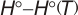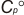.
| squib |
reference |
DOI |
| 1960Mil/Mor:1523 |
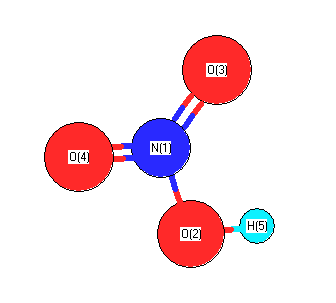
Millen, D.J.; Morton, J.R. "The Microwave Spectrum of Nitric Acid." J. Chem. Soc. 1960, 1523-1528 (1960) |
10.1039/jr9600001523 |
| 1965Cox/Riv:3106 |
Cox, A. Peter, Riveros, Jose M., Microwave Spectrum and Structure of Nitric Acid, J. of Chem. Phys., Vol. 42, #9 pgs. 3106-3112 |
10.1063/1.1696387 |
| 1979HUB/HER |
Huber, K.P.; Herzberg, G., Molecular Spectra and Molecular Structure. IV. Constants of Diatomic Molecules, Van Nostrand Reinhold Co., 1979 |
10.1007/978-1-4757-0961-2 |
| Gurvich |
Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub. Co., New York, 1989 |
|
| NSRDS-NBS10 |
R. D. Nelson Jr., D. R. Lide, A. A. Maryott "Selected Values of electric dipole moments for molecules in the gas phase" NSRDS-NBS10, 1967 |
10.6028/NBS.NSRDS.10 |
| Shim |
Shimanouchi, T. , Tables of Molecular Vibrational Frequencies, Consolidated Volu |
10.6028/NBS.NSRDS.39 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |