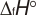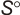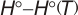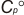Geometric Data
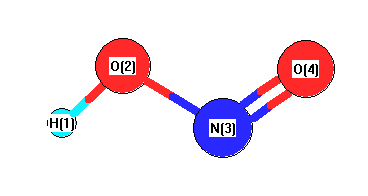
Point Group Cs
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| H1 |
1.7545 |
-0.1823 |
0.0000 |
| O2 |
0.8942 |
-0.6061 |
0.0000 |
| N3 |
0.0000 |
0.5252 |
0.0000 |
| O4 |
-1.1135 |
0.1693 |
0.0000 |
Atom - Atom Distances 
Distances in Å
| |
H1 |
O2 |
N3 |
O4 |
| H1 |
|
0.9590 | 1.8918 | 2.8895 |
| O2 |
0.9590 |
|
1.4420 | 2.1522 |
| N3 |
1.8918 | 1.4420 |
|
1.1690 |
| O4 |
2.8895 | 2.1522 | 1.1690 |
|
Calculated geometries
for HNO
2 (Nitrous acid).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| H1 |
O2 |
N3 |
102.100 |
|
O2 |
N3 |
O4 |
110.600 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-O |
1 |
| N-O |
1 |
| N=O |
1 |
Connectivity
| Atom 1 |
Atom 2 |
| H1 |
O2 |
| O2 |
N3 |
| N3 |
O4 |