.
| squib |
reference |
DOI |
| 1979HUB/HER |
Huber, K.P.; Herzberg, G., Molecular Spectra and Molecular Structure. IV. Constants of Diatomic Molecules, Van Nostrand Reinhold Co., 1979 |
10.1007/978-1-4757-0961-2 |
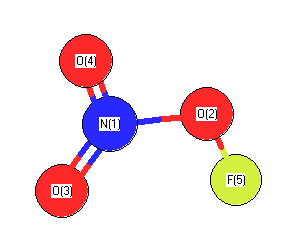
| 1994Cas/Dix:8317 |
Casper, B., Dixon, D., Mack, H., Ulic, S., Willner, H., Oberhammer, H., Molecular Structure of Fluorine Nitrate: Dangerous for Experiment and Theory, J. Am. Chem. Soc., Vol. 116, pgs. 8317-8321 |
10.1021/ja00097a044 |
| Gurvich |
Gurvich, L.V.; Veyts, I. V.; Alcock, C. B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub. Co., New York, 1989 |
|
| Shim |
Shimanouchi, T. , Tables of Molecular Vibrational Frequencies, Consolidated Volu |
10.6028/NBS.NSRDS.39 |