Geometric Data
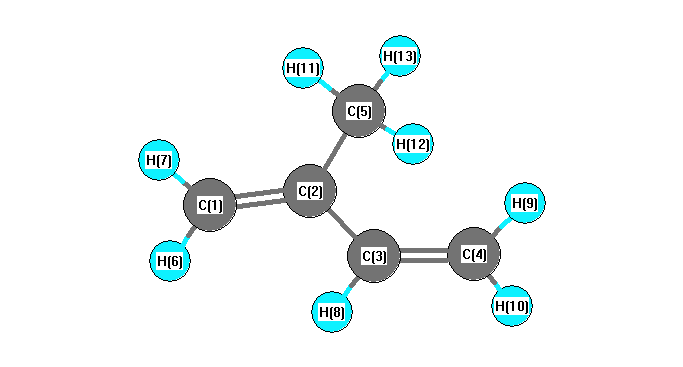
Point Group Cs
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
Cartesians
| Atom |
x (Å) |
y (Å) |
z (Å) |
| C1 |
0.5485 |
1.7492 |
0.0000 |
| C2 |
0.0000 |
0.5266 |
0.0000 |
| C3 |
0.8273 |
-0.6800 |
0.0000 |
| C4 |
0.4073 |
-1.9525 |
0.0000 |
| C5 |
-1.5013 |
0.3467 |
0.0000 |
| H6 |
1.6077 |
1.9386 |
0.0000 |
| H7 |
-0.0142 |
2.6663 |
0.0000 |
| H8 |
1.8660 |
-0.3990 |
0.0000 |
| H9 |
-0.6268 |
-2.2497 |
0.0000 |
| H10 |
1.0614 |
-2.8069 |
0.0000 |
| H11 |
-1.9867 |
1.3449 |
0.0000 |
| H12 |
-1.7995 |
-0.2173 |
0.9084 |
| H13 |
-1.7995 |
-0.2173 |
-0.9084 |
Atom - Atom Distances 
Distances in Å
| |
C1 |
C2 |
C3 |
C4 |
C5 |
H6 |
H7 |
H8 |
H9 |
H10 |
H11 |
H12 |
H13 |
| C1 |
|
1.3400 | 2.4452 | 3.7044 | 2.4837 | 1.0760 | 1.0760 | 2.5201 | 4.1681 | 4.5849 | 2.5673 | 3.1946 | 3.1946 |
| C2 |
1.3400 |
|
1.4630 | 2.5124 | 1.5120 | 2.1397 | 2.1397 | 2.0830 | 2.8462 | 3.4984 | 2.1486 | 2.1486 | 2.1486 |
| C3 |
2.4452 | 1.4630 |
|
1.3400 | 2.5448 | 2.7324 | 3.4505 | 1.0760 | 2.1397 | 2.1397 | 3.4668 | 2.8176 | 2.8176 |
| C4 |
3.7044 | 2.5124 | 1.3400 |
|
2.9881 | 4.0720 | 4.6380 | 2.1309 | 1.0760 | 1.0760 | 4.0748 | 2.9506 | 2.9506 |
| C5 |
2.4837 | 1.5120 | 2.5448 | 2.9881 |
|
3.4928 | 2.7553 | 3.4488 | 2.7397 | 4.0635 | 1.1100 | 1.1100 | 1.1100 |
| H6 |
1.0760 | 2.1397 | 2.7324 | 4.0720 | 3.4928 |
|
1.7778 | 2.3518 | 4.7471 | 4.7768 | 3.6432 | 4.1330 | 4.1330 |
| H7 |
1.0760 | 2.1397 | 3.4505 | 4.6380 | 2.7553 | 1.7778 |
|
3.5961 | 4.9540 | 5.5779 | 2.3742 | 3.5110 | 3.5110 |
| H8 |
2.5201 | 2.0830 | 1.0760 | 2.1309 | 3.4488 | 2.3518 | 3.5961 |
|
3.1047 | 2.5387 | 4.2290 | 3.7807 | 3.7807 |
| H9 |
4.1681 | 2.8462 | 2.1397 | 1.0760 | 2.7397 | 4.7471 | 4.9540 | 3.1047 |
|
1.7778 | 3.8432 | 2.5162 | 2.5162 |
| H10 |
4.5849 | 3.4984 | 2.1397 | 1.0760 | 4.0635 | 4.7768 | 5.5779 | 2.5387 | 1.7778 |
|
5.1505 | 3.9643 | 3.9643 |
| H11 |
2.5673 | 2.1486 | 3.4668 | 4.0748 | 1.1100 | 3.6432 | 2.3742 | 4.2290 | 3.8432 | 5.1505 |
|
1.8167 | 1.8167 |
| H12 |
3.1946 | 2.1486 | 2.8176 | 2.9506 | 1.1100 | 4.1330 | 3.5110 | 3.7807 | 2.5162 | 3.9643 | 1.8167 |
|
1.8167 |
| H13 |
3.1946 | 2.1486 | 2.8176 | 2.9506 | 1.1100 | 4.1330 | 3.5110 | 3.7807 | 2.5162 | 3.9643 | 1.8167 | 1.8167 |
|
Calculated geometries
for C
5H
8 (1,3-Butadiene, 2-methyl-).
Experimental Bond Angles (degrees) from cartesians 
| atom1 |
atom2 |
atom3 |
angle |
|
atom1 |
atom2 |
atom3 |
angle |
| C1 |
C2 |
C3 |
121.400 |
|
C1 |
C2 |
C5 |
121.000 |
| C2 |
C1 |
H6 |
124.300 |
|
C2 |
C1 |
H7 |
124.300 |
| C2 |
C3 |
C4 |
127.300 |
|
C2 |
C3 |
H8 |
109.300 |
| C2 |
C5 |
H11 |
109.100 |
|
C2 |
C5 |
H12 |
109.100 |
| C2 |
C5 |
H13 |
109.100 |
|
C3 |
C2 |
C5 |
117.600 |
| C3 |
C4 |
H9 |
124.300 |
|
C3 |
C4 |
H10 |
124.300 |
| C4 |
C3 |
H8 |
123.400 |
|
H6 |
C1 |
H7 |
111.400 |
| H9 |
C4 |
H10 |
111.400 |
|
H11 |
C5 |
H12 |
109.840 |
| H11 |
C5 |
H13 |
109.840 |
|
H12 |
C5 |
H13 |
109.840 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type |
Count |
| H-C |
8 |
| C-C |
2 |
| C=C |
2 |
Connectivity
| Atom 1 |
Atom 2 |
| C1 |
C2 |
| C1 |
H6 |
| C1 |
H7 |
| C2 |
C3 |
| C2 |
C5 |
| C3 |
C4 |
| C3 |
H8 |
| C4 |
H9 |
| C4 |
H10 |
| C5 |
H11 |
| C5 |
H12 |
| C5 |
H13 |
Dipole, Quadrupole and Polarizability
Electric dipole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Dipole (Debye) |
Reference |
comment |
Point Group |
Components |
| x |
y |
z |
total |
dipole |
quadrupole |
| 1 |
1 |
1A' |
Cs |
True |
0.250 |
0.035 |
|
0.252 |
NISThydrocarbon |
x=b, y=a |
Cs |
2 |
3 |
Experimental dipole measurement abbreviations: MW microwave; DT Dielectric with Temperature variation; DR Indirect (usually an upper limit); MB Molecular beam
Calculated electric dipole moments for
C
5H
8 (1,3-Butadiene, 2-methyl-).
Electric quadrupole moment 
| State |
Config |
State description |
Conf description |
Exp. min. |
Quadrupole (D Å) |
Reference |
comment |
Point Group |
Components |
| xx |
yy |
zz |
dipole |
quadrupole |
| 1 |
1 |
1A' |
Cs |
True |
1.700 |
3.300 |
-5.000 |
1971Fly/Ben:225 |
Qxx=1.7+-2.2, Qyy=3.3+-2.3, Qzz=-5.0+-3.2 |
Cs |
2 |
3 |
Calculated electric quadrupole moments for
C
5H
8 (1,3-Butadiene, 2-methyl-).











