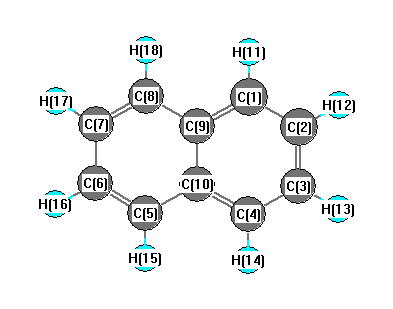
.
| squib |
reference |
DOI |
| 1976Hellwege(II/7) |
Hellwege, KH and AM Hellwege (ed.). Landolt-Bornstein: Group II: Atomic and Molecular Physics Volume 7: Structure Data of Free Polyatomic Molecules. Springer-Verlag. Berlin. 1976. |
|
| 1996Mar/ElY:15358 |
JML Martin, J El-Yazal, J-P Francois "Structure and Vibrational Spectrum of Some Polycyclic Aromatic Compounds Studied by Density Functional Theory. 1. Naphthalene, Phenanthrene, and Anthracene" J. Phys. Chem. 1996, 100, 15358-15367 |
10.1021/jp960598q |
| 1998Gus/Rui:163 |
M Gussoni, R Rui, G Zerbi "Electronic and relaxation contribution to linear molecular polarizability. An analysis of the experimental values" J. Mol. Struct. 447 (1998) 163-215 |
10.1016/S0022-2860(97)00292-5 |
| 2003Kab/Kas:3691 |
MH Kabir, S Kasahara, W Demtroder, Y Tatamitani, A Doi, H Kato, M Baba "Doppler-free laser polarization and optical-optical double resonance polarization labeling spectroscopies of a large molcule: Naphthalene" J. Chem. Phys. 119(7), 3691, 2003 |
10.1063/1.1590961 |
| webbook |
NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) |
10.18434/T4D303 |